Atomic and physical properties of the Group 1 elements
This section explores the trends in some atomic and physical properties of the Group 1 elements , also known as alkali metals – lithium, sodium, potassium, rubidium, and caesium.
Trends in Atomic Radius

As we move from lithium to caesium in the periodic table, each element gains an additional electron shell. This increase in electron shells results in a larger atomic volume and thus, a larger atomic and ionic radius.
Atomic Radii Trend:
- Li < Na < K < Rb < Cs
Physical Properties of Alkali Metals
Let’s take a closer look at some of the key physical properties of these elements.
| Property | Li | Na | K | Rb | Cs |
|---|---|---|---|---|---|
| Atomic Weight | 6.94 | 22.99 | 39.1 | 85.47 | 132.91 |
| Atomic Volume (cm³/mol) | 12.97 | 23.63 | 45.36 | 55.8 | 69.95 |
| Atomic Radius (Å) | 1.52 | 1.86 | 2.31 | 2.44 | 2.62 |
| Covalent Radius (Å) | 1.23 | 1.54 | 2.03 | 2.16 | 2.36 |
| Ionic Radius (Å) | 0.6 | 0.95 | 1.33 | 1.48 | 1.69 |
| Melting Point (°C) | 180.5 | 97.8 | 63.7 | 38.9 | 28.7 |
| Boiling Point (°C) | 1330 | 892 | 774 | 688 | 670 |
| 1st Ionization Energy (kJ/mol) | 520.3 | 495.8 | 418.9 | 403.0 | 375.7 |
| 2nd Ionization Energy (kJ/mol) | 7298.1 | 4562.4 | 3051.4 | 2633.0 | 2230.0 |
| Standard Oxidation Potential | 3.04 | 2.71 | 2.93 | 2.94 | 2.92 |
| Sublimation energy (eV/ion) | 1.7472 | 1.2432 | 1.032 | 0.984 | 0.9024 |
| Hydration energy (eV/ion) | 5.0904 | 3.792 | 3.6955 | 3.36 | 0.624 |
| Electronegativity | 0.98 | 0.93 | 0.82 | 0.82 | 0.79 |
| Color of the flame | Crimson Red | Golden Yellow | Lilac (Pink) | Violet Red | Blue/Violet (Indigo) |
| Enthalpy of atomization (kJ/mol.) | 159 | 107 | 89 | 80.9 | 76 |
| Density (g/cm³) | 0.534 | 0.972 | 0.859 | 1.525 | 1.903 |
Trends in Ionization Energy
First Ionization Energy: The energy required to remove the most loosely held electron from an atom decreases as we move down the group. This is due to the increasing distance of the outermost electron from the nucleus, making it easier to remove.
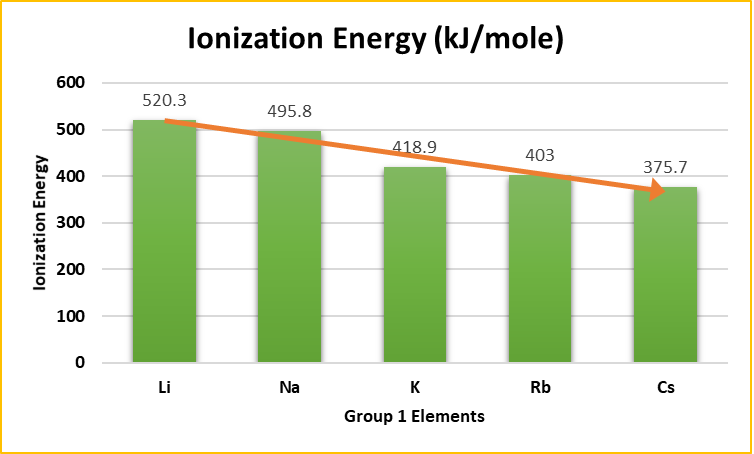
Trend:
- Li > Na > K > Rb > Cs
Alkali metals have low ionization energies due to their single ns¹ electron configuration. This outer electron is only weakly attracted to the nucleus, making it easy to remove.
Trends in Electronegativity
Electronegativity measures an atom’s ability to attract and hold onto electrons. In alkali metals, the outer electron is loosely held and can easily be excited to higher energy levels.
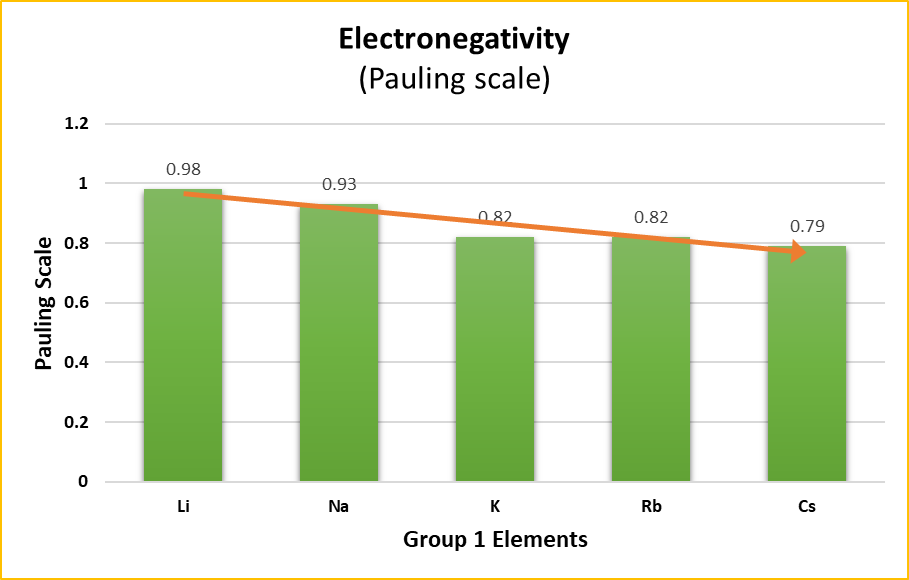
Trend:
- Li > Na > K > Rb > Cs
Flame Tests and Alkali Metals: Why Fireworks Sparkle
Alkali metals have an electron (ns¹) that’s easily excited by heat from a flame. This “jumped-up” electron eventually falls back to its original spot, releasing energy as a burst of colored light. Different metals emit different colors based on the energy gap between levels. We use this unique light signature (flame test) to identify alkali metals in unknown samples!

Each alkali metal produces a distinct flame color:
- Lithium: Crimson or a fiery red.
- Sodium: A bright, golden yellow. (Think: the iconic street lamp glow!)
- Potassium: A delicate lilac, pink, or pale violet.
- Rubidium: A transition from red to violet.
- Cesium: Blue transitioning to violet
Trends in Melting and Boiling Points
Both melting and boiling points decrease as we move down the group. The reason lies in the weak inner atomic bonds and the increase in atomic size, which reduces the effectiveness of the bonds holding the solid structure together.
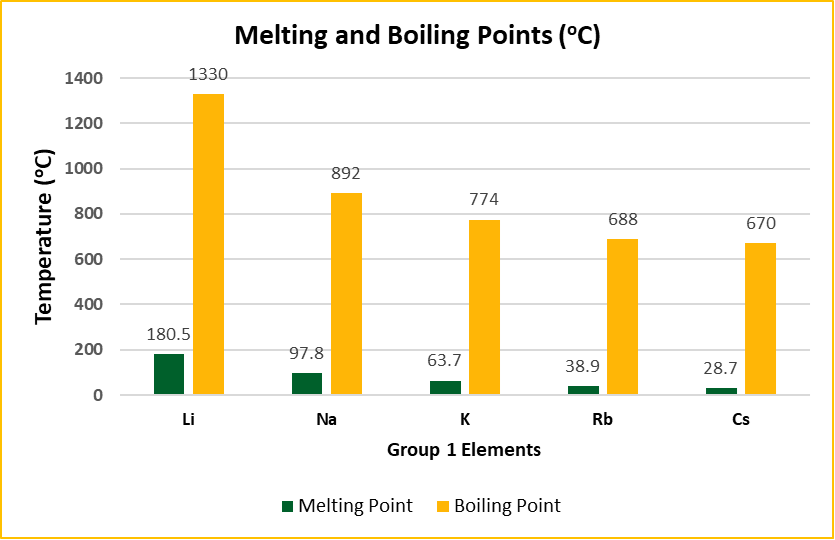
Melting Points:
- Li > Na > K > Rb > Cs
Boiling Points:
- Li > Na > K > Rb > Cs
Trends in Density
The densities of alkali metals are relatively low due to their large atomic volumes. Interestingly, potassium is lighter than sodium, which is an anomaly due to its larger atomic size.
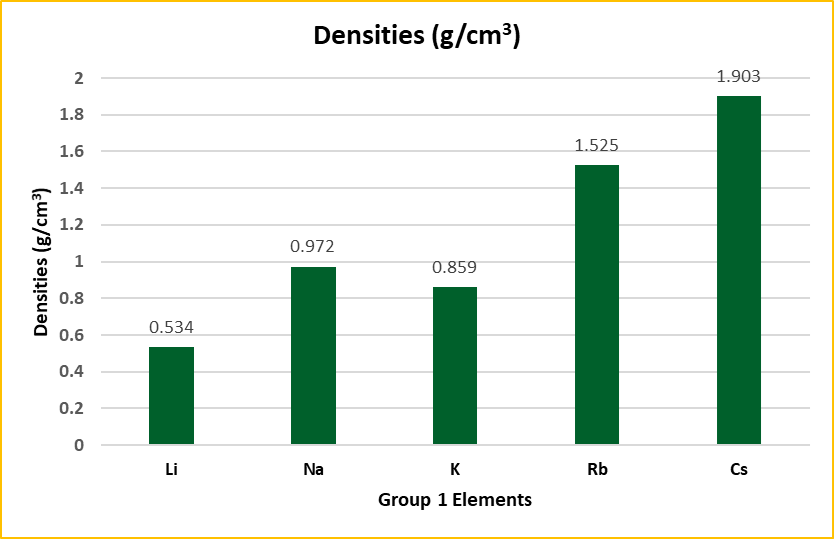
Densities at 0°C (g/cm³):
- Li: 0.534
- Na: 0.972
- K: 0.859
- Rb: 1.525
- Cs: 1.903

