Physical Properties of the Oxides of Period 3 Elements
This section looks at how the structure of oxides formed by elements in Period 3 (except Argon) affects their physical properties.
Overview of Trends
The Oxides to Consider:
| Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO3 | Cl2O7 |
|---|---|---|---|---|---|---|
| P4O6 | SO2 | Cl2O |
The oxides in the top row represent the highest oxidation states of the elements, where all outer electrons participate in bonding.
Structural Trends
The structure varies from metallic oxides with giant ionic lattices on the left to giant covalent (silicon dioxide) in the middle and molecular oxides on the right.
Melting and Boiling Points
- Giant Structures: The metal oxides and silicon dioxide exhibit high melting and boiling points due to strong ionic or covalent bonds requiring significant energy to break.
- Molecular Oxides: Phosphorus, sulfur, and chlorine oxides consist of discrete molecules. Their intermolecular forces (Van der Waals dispersion forces and dipole-dipole interactions) are weaker, leading to lower melting and boiling points, often resulting in gases or low-melting solids.
Electrical Conductivity
None of these oxides conduct electricity in the solid state as they lack free or mobile electrons. However, ionic oxides can conduct electricity when molten due to ion movement.
-
- Metallic Oxides (Sodium, Magnesium, Aluminium): These oxides, like sodium chloride, have giant ionic structures.
- Giant Covalent Oxides (Silicon Dioxide): Silicon dioxide has a structure similar to diamond, with each silicon atom bonded to oxygen atoms, forming a giant covalent network.
- Molecular Oxides (Phosphorus, Sulfur, Chlorine):
Phosphorus Oxides
Phosphorus forms two common oxides:
-
-
-
-
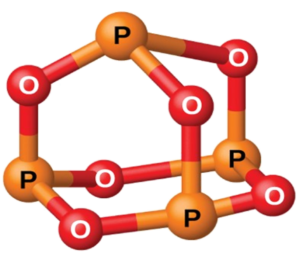
- Phosphorus(III) Oxide (P4O6): A white solid that melts at 24°C and boils at 173°C. The structure is based on a tetrahedral P4 molecule.
A tetrahedral P4 molecule P₄O₆ adopts a cage-like structure with four phosphorus atoms in a tetrahedral arrangement. Each phosphorus forms three single bonds with oxygen atoms, while the remaining oxygen atoms bridge each phosphorus pair with single P-O-P bonds - Phosphorus(V) Oxide (P4O10): A white solid that sublimes at 300°C, involving all five outer electrons in bonding, forming various polymeric structures.
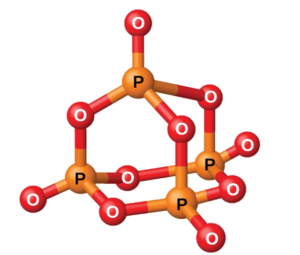
In P₄O₁₀, a network of phosphorus atoms is linked by oxygen atoms. Each phosphorus forms four bonds with oxygen, one a double bond and the remaining three single bonds.
- Phosphorus(III) Oxide (P4O6): A white solid that melts at 24°C and boils at 173°C. The structure is based on a tetrahedral P4 molecule.
-
-
-
Sulfur Oxides
Sulfur forms two common oxides:
- Sulfur Dioxide (SO2): A colorless gas with a choking smell, consisting of bent SO2 molecules due to lone pairs on the sulfur.
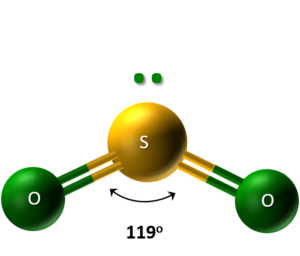
SO2 bends due to lone electron pairs on the sulfur atom, pushing the oxygen atoms closer and creating a 119° bond angle. - Sulfur Trioxide (SO3): Solid or Gas?
Sulphur trioxide (SO₃) exists as a white solid with low melting and boiling points. In the gas phase, SO₃ consists of simple molecules with all six outer electrons from sulphur involved in bonding.
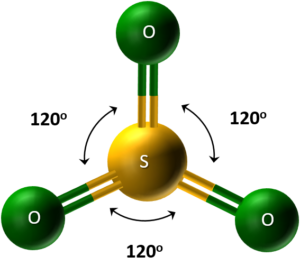
-
- Solid forms: Interestingly, SO₃ has various solid forms. The simplest is a trimer (S₃O₉), where three SO₃ molecules link together in a ring structure.
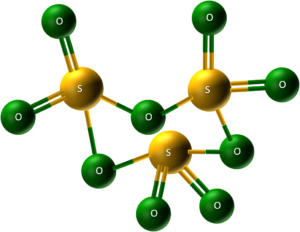
In (S₃O₉) trimer three SO₃ molecules link together in a ring structure. - Polymers: Other solid forms involve SO₃ molecules forming long chains, like polymers.
- Solidity explained: This tendency of SO₃ molecules to join and form larger structures explains why it’s a solid at room temperature, rather than a gas like its simple molecules.
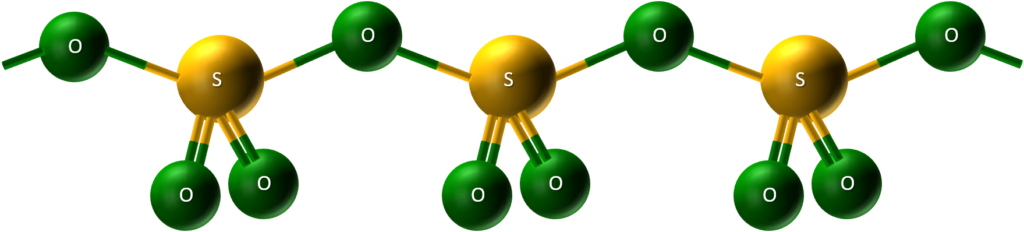
SO₃ forms long chains by linking its molecules together. This chain formation creates a stronger structure, making SO₃ a solid at room temperature instead of a gas.
- Solid forms: Interestingly, SO₃ has various solid forms. The simplest is a trimer (S₃O₉), where three SO₃ molecules link together in a ring structure.
Chlorine Oxides
Chlorine forms several oxides, including:
- Chlorine(I) Oxide (Cl2O): A yellowish-red gas at room temperature, involving one outer electron in bonding.
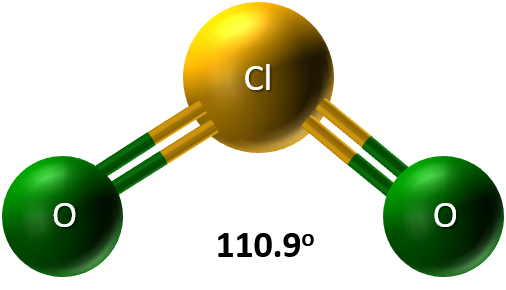
Cl2O features simple, small molecules where chlorine uses its single outer electron to bond with oxygen. - Chlorine(VII) Oxide (Cl2O7): A colorless oily liquid at room temperature, with chlorine using all seven outer electrons to bond with oxygen.
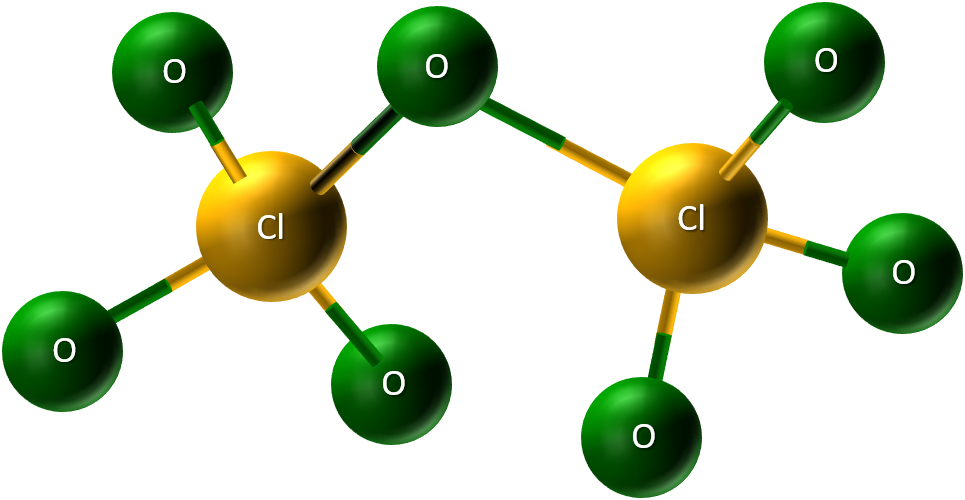
Cl2O7: Chlorine utilizes all seven of its outer electrons to bond with oxygen atoms, resulting in a much larger and more complex molecule compared to chlorine (I) oxide.